how to prepare edta solution for water hardness test|edta titrimetric method : sourcing In this experiment you will standardize a solution of EDTA by titration against a standard solution made from calcium carbonate, CaCO 3. You will then use the EDTA solution to . Resultado da Game summary of the La Equidad vs. Millonarios Colombian Primera A game, final score 2-1, from 9 November 2023 on ESPN (UK).
{plog:ftitle_list}
webGrupo de putaria Telegram. Melhores grupos de putaria reunidos em só lugar. Top grupos porno. O melhor da putaria.
Determination of hardness of water by EDTA method is one of the three main methods for determination of hardness of water. EDTA means Ethylenediaminetetraacetic acid. This EDTA reagent can be forming edta .Lab 1 Prepare and standardize your calcium carbonate and EDTA solutions. Lab 2 Analyze your water samples for total hardness and for calcium. Lab 3 Finish your analyses.
salts (abbreviated EDTA) form a chelated soluble complex when added to a solution of certain metal cations. If a small amount of a dye such as Eriochrome Black T or Calmagite is added to .
Adhesive Peel Tester purchaser
In this experiment you will standardize a solution of EDTA by titration against a standard solution made from calcium carbonate, CaCO 3. You will then use the EDTA solution to .Standardization of EDTA Prepare 0.01 M EDTA solution in 0.16 M Sodium Glycine Buffer: Add approximately 0.88 g of dry disodium EDTA salt and 25 mL glycine buffer solution to about 100 mL of DI water in a 250 mL beaker and swirl periodically. (Water used in the preparation of standard EDTA solutions must be totally free of polyvalent cations.500 mL bottle with three small portions of your EDTA solution, swirling to ensure the entire inner surface of the bottle has been rinsed, (to remove metals from the bottle’s inner surface), and transfer the rest of your EDTA solution to the bottle for storage. (Polyethylene is preferable to glass for storage because EDTA solutionsThe procedure is done in 3 steps 1) Preparation of a standard Ca2+ solution, 2) Standardi-zation of EDTA with the standard calcium solution and, 3) Analysis of an unknown Ca2+ sample with the standardized EDTA solution. 1. Standard calcium solution. 0.405 g of CaCO 3 is dissolved in HCl and diluted to a volume of 250.00 mL. What is the
In this experiment you will standardize a solution of EDTA by titration against a standard solution made from calcium carbonate, CaCO3. You will then use the EDTA solution to determine the hardness of an unknown water sample. Since .
water hardness edta titration calculations
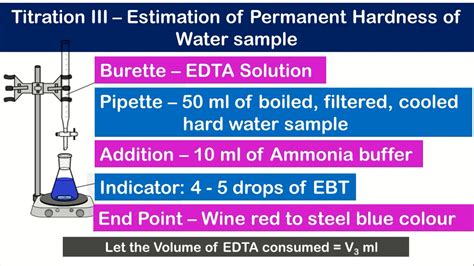
You will then use the EDTA solution to determine the hardness of an unknown water sample. Since both EDTA and Ca2+ are both colorless, . What is the concentration (molarity) of the EDTA solution? 2. If 100.00 mL of a water sample required 23.24 mL of EDTA of the concentration found in part e of problem 1, what is the hardness of the water in . Hardness Test apparatus Requirements. Take 50 ml water sample; Conical flask 250 ml; Ammonia buffer solution: Dissolve 67.5 gm of ammonium chloride in 570 ml of ammonium hydroxide and dilute to one liter with deionised water. Eriochrome Black -T indicator: Dissolve 0.5 gm of EBT in 10 ml Methanol & makeup to 100 ml 0.02 N EDTA Solution: Take .

A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.How to test water hardness & how to measure it using test strips, a water hardness soap test solution and other methods. . The modern way to test for hardness is by titration with EDTA (ethylenediaminetetraacetic acid), an organic chelating agent which reacts with both calcium and magnesium ions. When all calcium and magnesium ions are .
Preparation of a 0.0100 M EDTA Solution. Dry about 2 g of EDTA dihydrate, Na 2 H 2 Y 2 2H 2 O, in a drying oven at 80C for one hour. Then accurately weigh out about .95 g ± 0.lmg. Quantitatively transfer the EDTA into a 250 mL volumetric flask, add distilled water with mixing then dilute to the mark with distilled water.Mix well by inverting and shaking the tightly .
Complexometric titration is one of the best ways of measuring total water hardness. At pH around 10 EDTA easily reacts with both calcium and magnesium in the same molar ratio (1:1). Stability constant of calcium complex is a little bit higher, so calcium reacts first, magnesium later. . 0.01 M EDTA solution and ammonia pH 10.0 buffer. We will . Water Softening: Calcium (Ca2+) and magnesium (Mg2+) ions are responsible for water hardness. Metal-EDTA complexes with calcium and magnesium ions are water-soluble and prevent the formation of insoluble metal salts responsible for water hardness. Water softening is crucial for various industrial and domestic applications.
Hard water is caused by the presence of naturally occurring calcium and magnesium salts in water. Water hardness is usually noticed because of difficulty in lathering soap and the formation of a scum when washing. Calcium and magnesium ions (Ca2+ and Mg2+) form insoluble salts with soaps causing precipitation of this soap scum. Also, hard water .
Collect about 75 cm 3 of soap solution in a small beaker.; Set up a burette and, using the small funnel, fill it with soap solution. Use a measuring cylinder to measure out 10 cm 3 of one of the samples of water from the list below into a conical flask: . Rain water (solution A)Ammonia/Ammonium chloride buffer solution:6 Dissolve 6.75 g ammonium chloride (NH4 Cl) in 57 mL of 25% ammonia solution. Make up the solution to 100 mL with distilled water. Water hardness€is a measure of the amount of calcium and magnesium salts dissolved in water.7Total Water Hardness =[CaCO 3] = 2.5 * 24 mg/L + 4.1 * 28 mg/L = 174.80 mg/L The result is the total hardness of water: 174.80 mg/L or 174.80 ppm. If you have such a high water hardness, you might want to consider using a salt-free .
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula [CH 2 N(CH 2 CO 2 H) 2] 2.This white, slightly water-soluble solid is widely used to bind to iron (Fe 2+ /Fe 3+) and . Conditional Metal–Ligand Formation Constants. The formation constant for CdY 2– in equation 9.10 assumes that EDTA is present as Y 4–.Because EDTA has many forms, when we prepare a solution of EDTA .
Adhesive Peel Tester purchasers
Hardness, Calcium DOC316.53.01157 Titration Method with EDTA1, 2 Method 8222 0–25,000 mg/L as CaCO 3 Buret Titration Scope and application: For water, wastewater and seawater. 1 USEPA accepted when 0.020 N titrant is used. 2 Adapted from Standard Methods for the Examination of Water and Wastewater. Test preparation Before startingReagents: Buffer solution; EDTA Titrant; EBT 1. Measure Ca-Hardness and Total Hardness by titration as described below. Use a different sample for each measurement. 2. Total Hardness: Take 100 ml of the sample and add 2 ml buffer solution in it and add 2-3 drops of Black T. Titrate it with standard EDTA solution (with continuous stirring)500 mL bottle with three small portions of your EDTA solution, swirling to ensure the entire inner surface of the bottle has been rinsed, (to remove metals from the bottle’s inner surface), and transfer the rest of your EDTA solution to the bottle for storage. (Polyethylene is preferable to glass for storage because EDTA solutions A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.

Calmagite indicator and two chelating agents, EGTA and EDTA, are used in thetest. Chemical reactions Total hardness Several solutions including digital titrator cartridges are described in the following section for titrating prepared water samples containing calmagite indicator. TitraVer™ Hardness Titrant (0.020N EDTA) is the most widely used. This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern.
hardness of water procedure
hardness estimation by edta method
Chemists use an analytical technique called “complexometric titration” to analyze the amounts of dissolved metals in solutions. The technique typically involves placing the metal-containing solution in a beaker or flask and adding a complexing agent, such as ethylenediaminetetraacetic acid, or EDTA, dropwise from a buret.titrator. Drinking water, process water, cooling water, boiler water, wastewater, surface water, environmental water, raw water. Introduction Total Hardness in water is determined using the preprogrammed method, T7A Total Hard. To determine total hardness, ammonia buffer is added to a sample to adjust pH to 10.0. The sample is then titrated to the Ion Exchange Water Softener The Evidence. A 2005 study on water softeners for hardness removal concluded that a hardness concentration of up to 1000 mg/L could be removed by at least 81.68% by these systems.; Water softeners are also recommended by the EPA for homes in “areas of substantial hardness” thanks to their ability to remove the calcium . This concentration is determined by standardizing the titrant. How could you standardize the EDTA solution? The EDTA is standardized by titrating it was a solution of known Ca 2+ concentration. EBT is usually used as the indicator. Experimental analysis: The common procedure for this analysis is: Solutions needed: 0.00250 M EDTA solution
Adhesive Peel Tester purchasing
explain edta method in detail
WEBBrasfoot 2011 O gerenciador de futebol mais conhecido no Brasil em sua versão 2011. Arquivo seguro! O arquivo foi verificado e está alocado em nossos servidores. Iniciar Download (4.8 MB) Relacionados. Cannon Smash Um dos melhores jogos de tênis de mesa que existem. Freeride Earth
how to prepare edta solution for water hardness test|edta titrimetric method